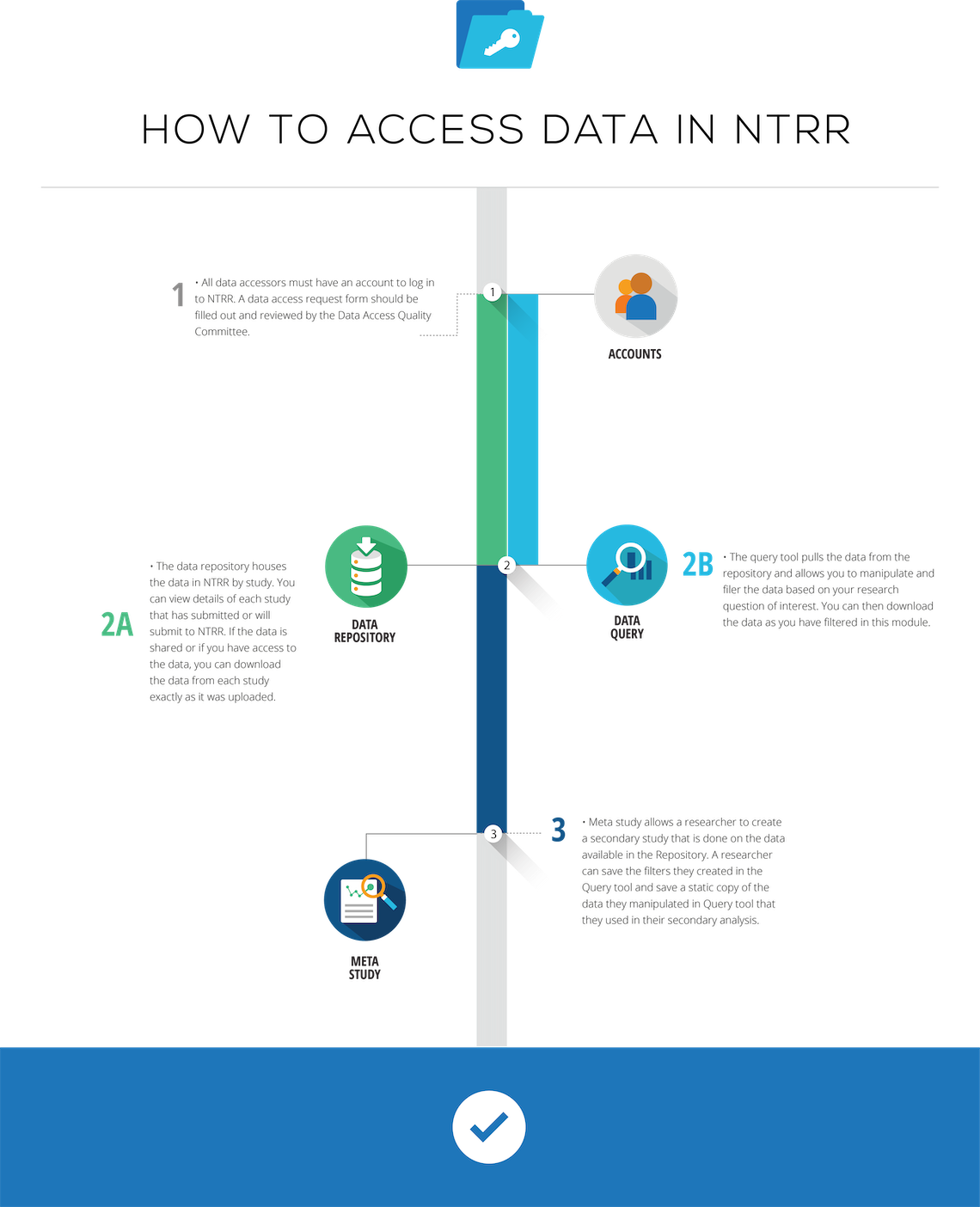
Qualified researchers can request access to data stored in the NTRR. To gain access to shared data, an investigator must obtain data access privileges.
The following criteria must be met in order to obtain access to NTRR data:
- Projects must be specified for research purposes only (not commercial) with the intention to enhance knowledge for the benefit of human health.
- Individuals requesting access must have a medical or scientific degree or position relevant for the request.
- Individuals must be affiliated with a research, industry, or non-profit institution/business/organization.
- All data access requests must be signed by an individual legally authorized to sign on behalf of the institution/business/organization.
Access may be denied or revoked if:
- The individual or affiliation cannot be confirmed.
- The request comes from a country or organization that is considered hostile or a security threat.
- There is reason to believe that the requester is not, or will not, adhere to the policy document.
In order to obtain Privileges, you must do the following:
- Complete the Data Access Request (includes Data Use Certification). This must be signed by the PI and Authorized Representative.
- Scan and save the signed Data Access Request (PDF) document.
- Click the LOG INTO NTRR button on the FITBIR homepage and click Request a NTRR account (if you already do not have a NTRR account) to submit data. NTRR account requests are reviewed and typically approved within 2 days.
- Fill in required fields. Be sure to select "Query Tool and Study" permission under Account Privileges.
- Upload required, signed documents. Select the File Type from the drop-down menu: Data Access Request
- Browse and select your signed PDF of the Data Use Certification (part of the Data Access Request Document)
- Click the Upload button
- Click SUBMIT REQUEST.
Once completed, the request package is then sent for approval to the Data Access and Quality Committee (DAQ) established to oversee access to NTRR shared data. When the investigator's request is approved, the investigator is notified by e-mail and explained the conditions under which the approval is granted.
Approvals for access to NTRR and its tools are granted for one year. In order to apply for account renewal to submit data, users are required to update and submit the data submission request and biographical sketch. Users who would like to renew access to the query tool are required to update and submit the data access agreement and biographical sketch. If the investigator publishes any new results based on analysis using NTRR data and tools, he/she is required to acknowledge both the original author/submitter of the data and the NTRR. Investigators who access data are also strongly encouraged to collaborate on data analysis and publications with the PIs who collected the data.
